Products Information
- Oxygen sensors
- Oxygen sensing technology
Oxygen Sensing Technology
The sensors for the measurement of Oxygen are electrochemical devices that generate a current output in proportion to the oxygen partial pressure in the surrounding gas. Similar to other electrochemical devices, such as a battery, a sensor is composed of a cathode, an anode and electrolyte.
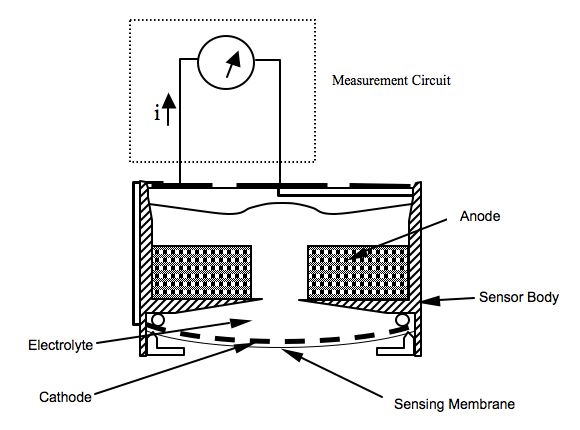
Oxygen supply to the cathode is precisely regulated by a membrane, which covers the surface of the cathode. During operation, the cathode and the anode are connected together. As the result, oxygen is electrochemically reduced at the cathode while the anode is oxidized and current flows through the external circuit in direction. The current magnitude is proportional to the rate of oxygen reaching the cathode through the sensing membrane, which in turn is a function of the partial pressure of oxygen outside the sensor.
Anode
(Pb + 2OH- → Pb2+ + H2O + 2e-)made of metallic Lead (Pb). It provides a suitable potential for oxygen being reduced at the cathode. It acts as fuel to be “burned” with oxygen. Therefore, an oxygen sensor with a Pb anode is self-powered, and known as Fuel-Cell type sensor. The oxygen reduction process on the cathode and the lead oxidation process on the anode (understood as a “burning” process) take place simultaneously inside the sensor. The lead oxidation produces lead ions (Pb2+) and two electrons per lead molecule.
Cathode:
(O2 + 2H2O + 4e- → 4OH-)the electrode where oxygen receives four electrons per molecule. Nothing but oxygen should react on the cathode. Therefore, an inert metal, such as platinum, gold or silver can be used as the cathode material. In fact, these noble metals catalyze the four-electron reduction for oxygen, making the sensor specific for oxygen measurement.